What is Biexponential and Hyperlog Scaling?
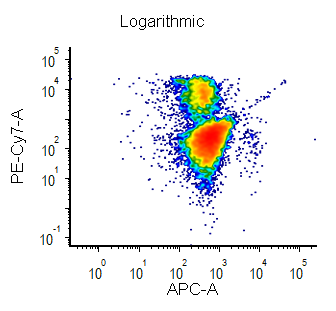
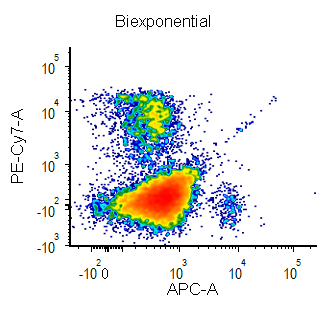
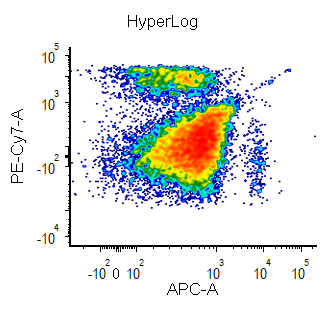
Biexponential and Hyperlog™ are equations that are characterized by exhibiting pseudo-linear like behavior values near a reflection point (usually zero) and transitioning to pseudo-logarithmic behavior at values distant from the reflection point. These equations are often used to display flow cytometry data with a high dynamic range (usually >16 bits) on a single plot. They are preferred to the logarithmic scale mainly for the following characteristics:
- values below zero can be displayed
- plots displayed in log can exhibit display artifacts on plots commonly used in flow cytometry (1024 channels). These artifacts are largely avoided in the Biexponential and Hyperlog displays.
 |
 |
 |
The FACSDiva™ software allows you to view a parameter on a biexponential scale by opening the inspector for the plot (View->Inspector) and setting the checkbox for the the axis you wish to show as biexponential, as in the image below.
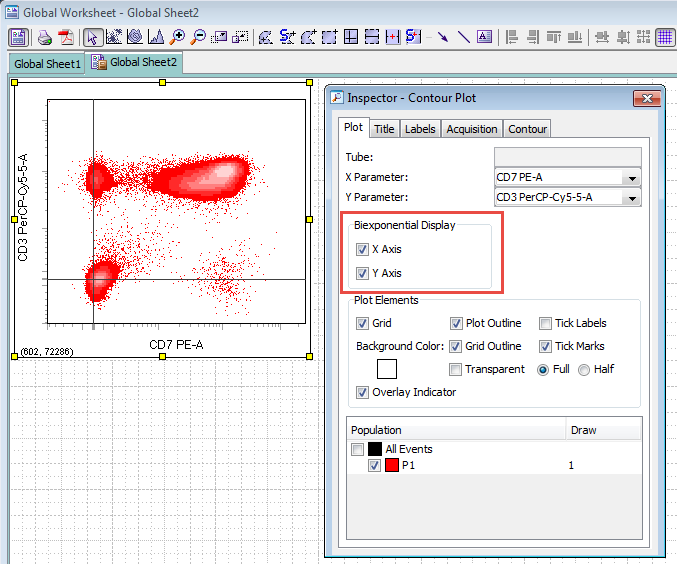
By default, the FACSDiva software calculates the reflection point automatically. FACSDiva uses a proprietary algorithm to calculate the reflection point (it is not the method suggested in the Parks et. al. paper, see Further Reading below) in order to optimize the biexponential display for data generated by BD instruments. The FACSDiva software does not tell you what reflection point it calculated, but it does tell you a "Below Zero" value. This Below Zero value can be considered to be approximately the lowest data value that will be plotted on your plot (this is not exactly correct, but is a close enough approximation). It is important to note that there may be data in your data file that is not actually plotted on the plot. This is particularly true if there are a few outlying events that are much more negative than the bulk of your population. The algorithm is designed to ignore these events, so that these few events do not unduly influence the entire plot. If they were not ignored, there could be a large amount of area on your graph devoted to just a few cells.
Thus, it is important to reiterate that, by default, the Below Zero value can change from data file to data file. That means that even though two plots may look similar, one may have to closely inspect axes to see if the axes vary widely from plot to plot. Please see the paper by Novo & Wood below for a more detailed explanation and examples.
It is possible to view the Below Zero value by opening the Biexponential Editor in the FACSDiva software (View->Biexponential Editor), as in the image below.
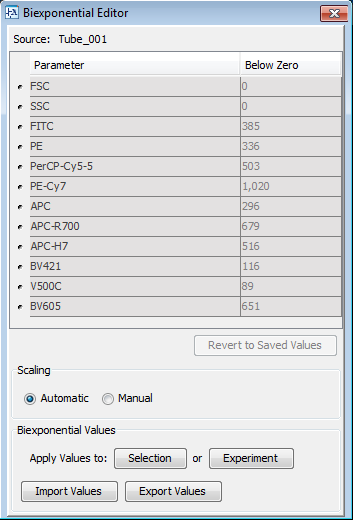
Note that the Below Zero value has been automatically calculated for each of the parameters in the data file. You can enter the Below Zero value yourself by selecting the Manual radio button and entering the values you desire. You would manually enter values if you wanted the axis scaling to be consistent from sample to sample.
By default, the Below Zero is calculated based on all events, however FACSDiva also allows you to choose the population that it will use to automatically calculate the Below Zero value. You can select this by right clicking on a plot and selecting Scale to Population and then choosing the population you want, as in the image below:
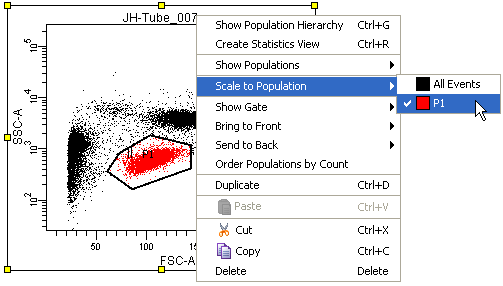
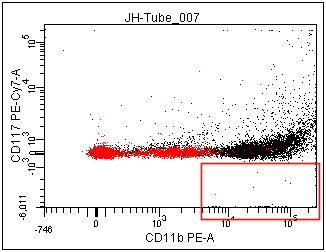
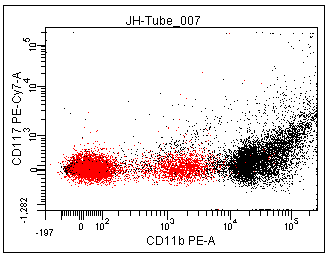
You can see in the two images below that the Scale to Population can have a dramatic effect. Setting the Scale to Population to a particular population will have the effect of trying to make the lowest cluster of data in that population appear as a cluster in the area of the plot immediately adjacent to the axis.
In the left panel below, notice the minimum value on the Y-axis is -6,011. This is due to there being some events (red box ) that are much more negative than the rest of the population. Most of these events are not in the P1 population. By changing the scale-to to be based on P1 (right panel) the Below Zero calculation does not consider the events that are outside P1, which includes most of the events in the red box. Thus, the Below Zero value is much more positive (only -1,282) in the panel on the right.
 |
 |
||
| Reflection point based on All events | Reflection point with Scale-to based on P1 (red) |
As a result of our close collaboration with BD, FCS Express now supports all of the options that FACSDiva does in terms of displaying biexponential data. In fact, we have implemented FACSDiva's proprietary algorithm for calculating the reflection point, so the results from FCS Express will look identical to the results from the FACSDiva software.
FCS Express supports a wide variety of scaling formulas.
To set the scaling on a plot, right-click on the plot and select Format from the popup menu that appears.
Select the Axis category, as in the image below. You can set the scaling independently for each axis, and choose from a wide variety of scaling formulas. The different formulas are explained in detail in the FCS Express manual.

Each scaling formula also has different options associated with it. These options appear immediately below the scaling selection menu when you select a particular scaling in FCS Express.

The Hyperlog scaling equation only has a single adjustable parameter, corresponding to the b variable in the Bagwell paper. This parameter can be thought of as the channel value below which the scaling exhibits predominantly linear-like behavior.
The biexponential options are more complicated, and are shown in the image below. We will describe each property in detail.
Certain data files, particularly those collected in BD FACSDiva, contain information within the file metadata to determine the extent of the data below zero.
However, the properties of the biexponential scaling can be customized as needed.
When the Automatic checkbox is checked the extent of negativity for your data will be set based on the metadata contained within your data.
When the Automatic checkbox is checked, all of the specific biexponential options are disabled. In order to enable the different options, uncheck the Automatic checkbox.
When the Automatic checkbox is unchecked, the plot will use the biexponential options from the Format screen to customize the data display.
If you select the Use this value radio button (as seen in this image), the plot will use the value that you entered (in the image, 1000). Selecting a fixed Below Zero value can be used with a combination of modifying the minimum resolution of the plot if you wish for the axis scaling on the plot to be consistent from sample to sample.

New to FCS Express 7, slider bars allow you to customize the extent of negativity based on a visual preference, rather than by entering a number.

Simply slide the bar either to the left or right to adjust the extent of negativity for that axis one way or the other.
If you wish to have the extent of negativity adjust automatically based on the data, you can select the Calculate automatically radio button. This will mimic the default behavior in FACSDiva.

When you choose to calculate automatically you will be give two additional choices.
- With the gate allows you to choose which gate you want to use to base the extent of negativity. This is identical to the Scale to Population feature in the FACSDiva software, described above. You can choose to base the calculation on the gate that is applied to the plot, you can choose No Gate, or you can select a specific gate that is defined in the layout.
- Note: selecting "No Gate" is equivalent to having the extent of negativity based on All Events in FACSDiva, which is the default behavior in FACSDiva.
- With the method allows you to choose which method to use to calculate the extent of negativity. The two choices are:
- Fifth Percentile: This is the method described in the Parks et al. paper. It considers the Below Zero value to be the 5th percentile of the negative values within the gate being considered.
- BD FACSDiva method: This is the method used in the FACSDiva software. It is proprietary to BD and provides optimal data display for data acquired on BD instruments.
When you open a plot in FCS Express with a FACSDiva data file, using FCS Express' default User Options, the data display will be similar as you were looking at it in FACSDiva - in linear, log or biexponential, and using the same biexponential Below Zero values.
In most cases, FCS Express will show the plot using the previously calculated value from FACSDiva. However, if you had chosen a Scale to Population in FACSDiva, FCS Express will not be able to match the values, because they are not stored in the data file. In that case, you will be able to recreate the way it looked by using the formatting options described above.
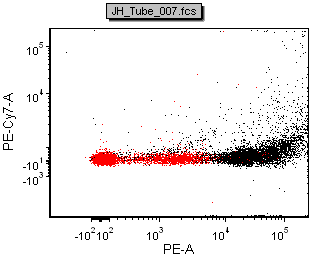
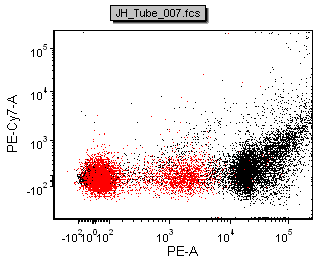
The figure below shows a comparison of the data from FACSDiva and FCS Express
 |
 |
||
| Scale-to All Events in FACSDiva | Scale-to based on P1 in FACSDiva | ||
 |
 |
||
| Scale-to All Events in FCS Express | Scale-to based on P1 in FCS Express |
The BD FACSDiva importer in FCS Express will import your experiments from the FACSDiva software and recreate a similar analysis in FCS Express. More information can be found here. Prior to FCS Express version 3.00.0800, plots that were shown in biexponential in FACSDiva were imported as hyperlog in FCS Express. However, versions subsequent to 3.00.0800 will preserve the biexponential scaling when the experiments are imported into FCS Express. (To check your version number, go to Help->About from the main menu). Unfortunately, if you selected a Scale-To Population in FACSDiva, FCS Express will not be able to set up the "with the gate" option described above to be the same as the scale-to population. This is because the FACSDiva exported XML file does not contain the name of the scale-to population that was chosen. You will have to go to the plots in FCS Express and select the proper gate yourself.
The following articles provide more detailed information than can be found in this web page:
A general review on scaling displays in flow cytometry:
Novo, D. & Wood, J (2008). Flow Cytometry Histograms:Transformations, Resolution and Display. Cytometry: 73A: 685-692.
A detailed description of the Hyperlog transformation:
Bagwell, CB. (2005). Hyperlog-a flexible log-like transform for negative, zero, and positive valued data. Cytometry. 64: 34-42.
A detailed description of the Biexponential transformation:
Parks DR, Roederer M, Moore WA. (2006). A new "Logicle" display method avoids deceptive effects of logarithmic scaling for low signals and compensated data. Cytometry. 69: 541-545
The key adjustable parameter of these equations is the transition point, i.e. at what point along the data does the equation transition from linear-like to log-like behavior.
Since the biexponential display was popularized by the BD FACSDiva software, we will begin our discussion there.
FACSDiva is a trademark of Becton Dickinson Inc
Hyperlog is a trademark of Verity Software
tags: bi-exponential, negative, plot, axis, axes
-
Installation
-
Licenses
-
- Can I get more information regarding the Add-Ons that can be purchased with a license?
- Can I lock my template based on an electronic signature?
- Does FCS Express have any features to help meet 21 CFR Part 11 compliance?
- Does FCS Express have Quality Control features?
- Does FCS Express offer Single Sign On capability?
- How do I configure SQL Server to host a database for FCS Express?
- What database options are available when I purchase the Security option?
- What is the difference between the different types of Users that are available with a Security and Logging license?
- What is the difference between the Logging option and System Level Audit Trails?
- What SQL Server permissions are needed?
-
-
- Can I share my USB dongle or countercode license with another user?
- Can I track usage of the internet dongle?
- Can I try out the Internet Dongle before I make a purchase?
- Can the administrator log users out?
- Do you have to be connected to the internet at all times with the Internet dongle?
- How can users be added to an internet dongle license?
- How do I activate my dongle?
- How do I change my internet dongle/site license password?
- How many people can be logged in at the same time?
- How many user accounts can I create?
- If a user left the computer running can the user log themselves out from another computer?
- What are the differences between the internet dongle and network licensing options?
- What happens if I lose my internet connection?
- What happens if the user leaves the computer without logging out?
- What happens to the users login in case of an unexpected interruption? For instance, a software crash, power failure, etc.
- Why am I receiving a message that FCS Express cannot connect to De Novo Software servers?
- Show all articles ( 1 ) Collapse Articles
-
-
- Can I convert my Cytek license from the countercode licensing option to another licensing option?
- How can I claim my license purchased through BD Accuri Cytometers?
- How can I claim my license purchased through BD Biosciences?
- How can I claim my license purchased through Nexcelom Biosciences?
- How can I claim my license purchased through Sysmex-Partec GmbH?
- How can I claim the FCS Express license that came with my Cytek instrument purchase?
-
-
Layouts & Loading Data
-
- Can I customize my Quick Access Toolbar?
- For an existing layout, can I change the way FCS Express remembers parameters?
- How can I exclude histogram overlays from batch processing?
- How do I change my layout from portrait to landscape?
- How do I change the resolution of my exported images?
- How do I re-establish data files that were linked to a layout?
-
- Are Beckman Coulter LMD files unique?
- Can I find a support resource page for the analysis of Cytek data in FCS Express?
- How can I easily create the "filename" column in the "ExtraKeywords Table.csv" file?
- How can I load a Sony MA900 Index Sort file into FCS Express?
- How can I load data from the BD Accuri C6 Flow Cytometer?
- How can I load MACSQuantify files that were exported from MACSQuantify software version 3.0.1?
- How can I quickly reload all of the data files in the data list?
- How do I change the display in my plots from one data file to another data file?
- How do I export .ICE files from Thermo Cellomics HCS Studio?
- How do I tell FCS Express what plate size to use if that information is not included in the data file?
- How do I upload files to the De Novo Software FTP site?
- How do I use BD Accuri CFlow files with Multicycle DNA analysis in FCS Express?
- How do I work with images from the Thermo Scientific Attune CytPix?
- What is the Elapsed Time setting in the Gallios software and how do I convert it to real time?
- Why am I seeing a warning message when loading my Cytek data onto a layout object?
- Why are iterations in my Data List gray?
- Why are there sometimes access violations when I save and load files?
- Why do I get the message that a data file exported from a FACSDiva™ Experiment is invalid?
- Show all articles ( 3 ) Collapse Articles
-
- How are existing quadrants handled when an old layout is opened in version 7.20 and later?
- How can I set quadrants to behave like conventional gates?
- How can I set quadrants to behave like in earlier versions?
- How can quadrants be linked?
- Quadrants in FCS Express versions 7.20 / 7.24 and later
- Why does the Quadrants Options window appear when I open an older layout in version 7.24?
- Why have percentages reported by quadrants changed after updating to FCS Express version 7.20.20?
-
-
Data Analysis
-
- Caveats when using Biexponential Scaling with automatic Below Zero parameter detection in the presence of outliers.
- How can I create a merged data with equally-sized downsampled samples?
- How can I do pre-processing for high-dimensional data analysis?
- How can I explore tSNE/UMAP plots?
- How do I use SPADE?
- What is FlowSOM?
- What is T-SNE?
- What is UMAP?
-
- Can FCS Express integrate Python scripts?
- Can I use the FlowAI script in FCS Express?
- Can I use the FlowClean R Script with FCS Express?
- How can I create a matrix of autofluorescence, and import autofluorescence into EasyPanel?
- How can I recreate ratiometric data acquired in FACSDiva?
- How do I use R Integration with FCS Express?
- How does FCS Express implement software compensation?
- If my data does not have a Time parameter, can I create one?
- What is compensation?
- What is the compensation workflow in FCS Express?
- When acquiring spectral data, should my single-stained controls be "as bright or brighter" than my fully-stained sample?
-
- Can a set of quadrants be both percentile and floating?
- Can I customize the display of my data from different instruments?
- Can I disable the live updating feature?
- How can I display all of my detectors for my Cytek data?
- How can I set FCS Express so my FCS 3.0 biexponential data looks the same as it did in the BD FACSDiva software?
- How do I display Summit data in FCS Express as it appears in the Summit Software?
- How do I fix the biexponential axes on a plot?
- How do I rescale CytoFLEX and DxFLEX data so it displays as it did at acquisition?
- How do I update my density and contour plots created in Version 4 to use the newest color palette?
- What are resolution options?
- What is Biexponential and Hyperlog Scaling?
- What is the best way to set FCS Express to display FCS 3.0 data from FACSDiva on a 4 decade log scale?
- Where can I get more information regarding DNA analysis using the Multicycle AV?
- Why can’t I change my plot axis labels from the Name keyword to the Stain keyword?
- Why do I see a warning message when inserting a Spectrum Plot?
- Why do my dot plots appear sparse and blocky?
- Why is the text on the right most label cut off my plot?
- Show all articles ( 2 ) Collapse Articles
-
- Can I create an output file that contains the same plot from each data file on a single page?
- Can I export spectral data to the FCS format?
- How can I successfully export a GatingML file?
- How do the batch processing run modes differ, and why would I use them?
- Why do I get an “Old format or invalid type library” error when using Microsoft excel during batch analysis?
-
- How are statistics in FCS Express calculated compared to how they are calculated in BD FACSDiva?
- How can I display my statistical data in Scientific Notation?
- How do I calculate EC/IC Anything?
- What is “Stain Index” and how do I calculate it with FCS Express?
- What is MFI (Mean or Median Fluorescence Intensity) and how do I calculate it in FCS Express?
- Why have percentages reported by quadrants changed after updating to FCS Express version 7.20.20?
- Why is the Geometric Mean being reported as NaN or ##ERROR##?
-
-
Image Cytometry
-
- How do I adjust the axes to display small particle data from Amnis CellStream?
- How do I choose which images and parameters to view in a Data Grid?
- How do I export/save data from IDEAS software and load it in FCS Express?
- How do I make my images in the data grid larger?
- How do I pseudo-color images in a data grid?
- How do I work with Amnis derived image cytometry data in FCS Express?
-
- Can I display heat maps with my Image Cytometry data?
- Can I work with data from PerkinElmer Instruments?
- Do you offer 21 CFR Part 11 compliance options for the Image Cytometry Version?
- Do you offer image segmentation or image analysis?
- How can Attune™ CytPix data sets with images (.ACS files) be merged for high dimensional analysis?
- How do I use CellProfiler Data with FCS Express?
- How do I use ImageJ with FCS Express?
- What file formats are compatible with FCS Express Image Cytometry?
- Where can I find Nexcelom Resources and Applications?
-
-
FCS Express on Mac
-
Upgrading FCS Express
-
- Can different versions of FCS Express exist on the same computer?
- How can I view and convert my V3 layouts to FCS Express 7?
- How do I import my version 3 security databases into newer versions of FCS Express?
- How do I update Density Plots created in Version 4?
- Is there an upgrade discount from earlier versions of FCS Express?
- Version 4 Internet Dongle Retirement
- Why are my density plots from V3 not displayed correctly in later versions?
- Why are there fewer outlier dots on my FCS Express 5 and later density plots than in V4?
-
Clinical & Validation Ready
-
- Can I get more information regarding the Add-Ons that can be purchased with a license?
- Can I lock my template based on an electronic signature?
- Does FCS Express have any features to help meet 21 CFR Part 11 compliance?
- What is the difference between the different types of Users that are available with a Security and Logging license?
- What is the difference between the Logging option and System Level Audit Trails?
 Explore the Scientific R&D Software
Explore the Scientific R&D Software