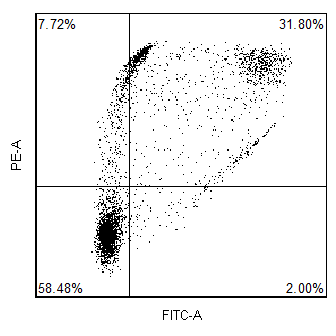
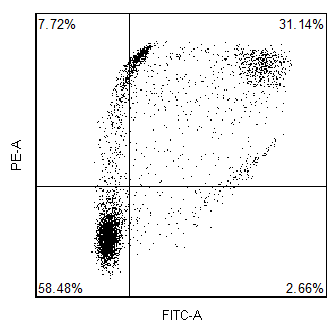
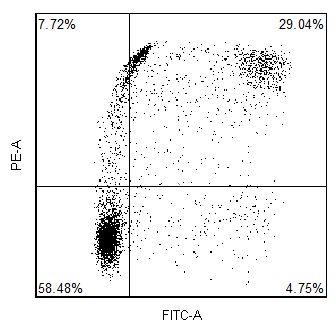
How does FCS Express implement software compensation?
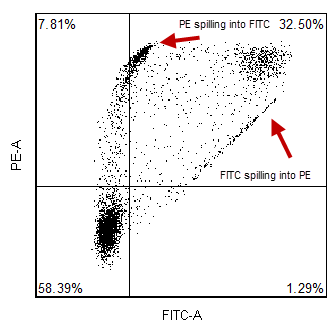
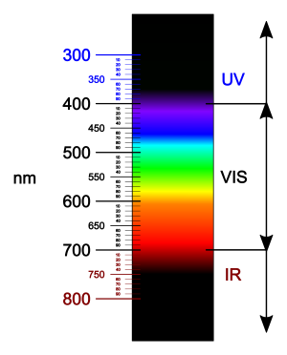 The emission spectrum (the wavelengths of light at which a given fluorophore emits) generally overlaps several detection channels of a typical commercial flow cytometer. Although most of the fluorescence signal will be recorded in a single primary channel, it is common that signal from the same fluorophore can be detected in two (or sometimes more) secondary channels. Compensation is the process of removing the unwanted fluorophore signal from secondary channels. A classic example is that although the fluorescence from fluorescein is primarily measured in the “green” channel (~530 nm), a significant amount of fluorescein fluorescence can also be detected in the “yellow” channel (~570 nm). Thus, if you are trying to detect another fluorochrome in the “yellow” channel (i.e. PE), the signal you observe in the “yellow” channel will comprise fluorescence from both PE and FITC
The emission spectrum (the wavelengths of light at which a given fluorophore emits) generally overlaps several detection channels of a typical commercial flow cytometer. Although most of the fluorescence signal will be recorded in a single primary channel, it is common that signal from the same fluorophore can be detected in two (or sometimes more) secondary channels. Compensation is the process of removing the unwanted fluorophore signal from secondary channels. A classic example is that although the fluorescence from fluorescein is primarily measured in the “green” channel (~530 nm), a significant amount of fluorescein fluorescence can also be detected in the “yellow” channel (~570 nm). Thus, if you are trying to detect another fluorochrome in the “yellow” channel (i.e. PE), the signal you observe in the “yellow” channel will comprise fluorescence from both PE and FITC
In many flow cytometers, compensation is built directly into electronics of the flow cytometer. This is typically called hardware compensation or analog compensation. There would be dials or buttons that would allow the user to change the compensation parameters before acquiring the data.
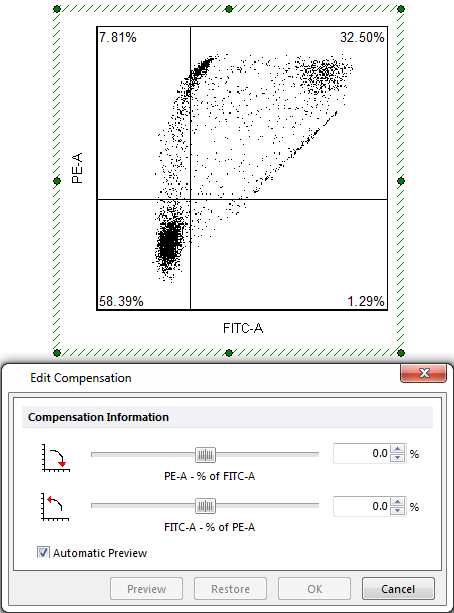
The dial would typically be labeled something like:. FL2-%FL1, or PE-A-% FITC-A. Where FL2 is the total amount of fluorescence observed or measured in the FL2 channel and the %FL1 is the percentage of fluorescence measured in the FL2 channel that is from the FL1 fluorochrome. By changing the dial or adjusting the setting, the user is changing the amount of fluorescence from the FL1 or FITC parameter to subtract from the total fluorescence measured in the FL2 or PE parameter. For example, if the FL2-%FL1 or PE-A-% FITC-A value was 20, this means that the true FL2 value is the observed FL2 value minus 20% of the observed FL1 value.
In addition to hardware compensation, the data can also be acquired uncompensated and then compensated in software. The advantage of this is that since you never compensate the raw data, you never have to worry that you acquired the data with the wrong compensation settings.
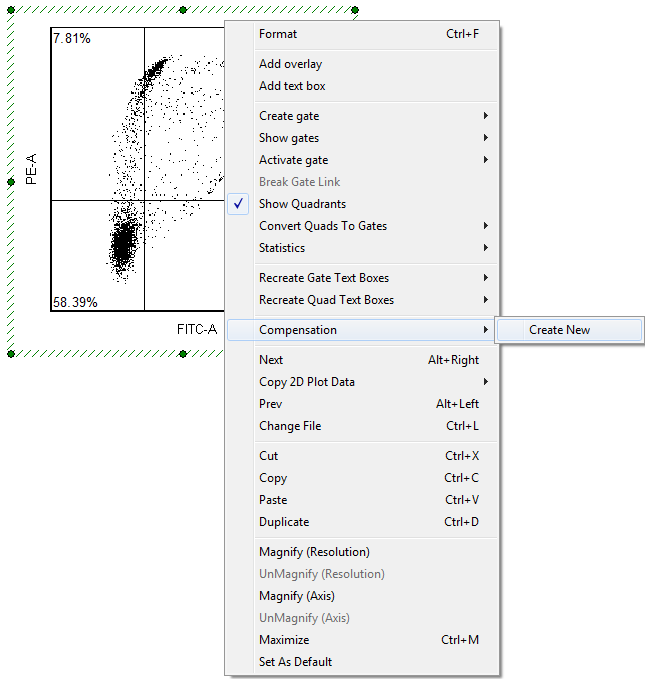
To use the automatic compensation feature of FCS Express it is necessary to have data from single stained positive control cells or from antibody capture beads for each parameter that requires compensation
Software compensation works by solving the system of equations described below:

Equation (1.5) can be written more succinctly as:
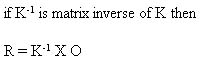
Thus, in order to find the real values for a given parameter, we have to use the inverse matrix. The inverse matrix is a set of coefficients that are multiplied by the observed signals. This is equivalent to FCS Express determining the value of the dials in manual compensation..
When you do automatic compensation in FCS Express you will see the inverted matrix K-1 (often called the compensation matrix) in the compensation screen . The non-inverted matrix K is often called the spillover matrix. The Compensation and the Spillover Matrix can be viewed in FCS Express. The spillover matrix is calculated internally from the means or medians of your single stained controls.
However, to reiterate, there is no difference between how the compensation matrix values are applied, and how manual values would be applied. That is why you can still adjust "automatic" compensation values using the sliders. It just gets very difficult to compensate more than a few parameters "by eye". Also, automatic compensation guarantees that the correct matrix coefficients are calculated based on your single stained controls.
That being said, there is an important difference between how compensation is usually implemented in hardware and the matrix method. In hardware compensation there is usually no way to change the coefficient from a single channel into itself.
from rearranging equation (1.3) you can see that
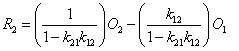
if either k21 or k12 is small, the first term will be negligible. However, if both k21 and k12 are large, this first term becomes important. That is why, in FCS Express, after you perform automatic compensation, you often find that kxx is not exactly 1.
In FCS Express you can look at the crossover matrix from the compensation screen. It displays the matrix as source and target values. Source is where the fluorescence is coming from, target is where the fluorescence is appearing. The kxx value appears where there source and target are the same. If you do not see a value where the source and target are the same, FCS Express is assuming that the crossover value for kxx is 100%.
Please be aware that you are not compensating properly if you change the values that FCS Express calculates for the compensation matrix, assuming the correct single stained and negative control data were used.
For a more practical discussion on how to set up automatic compensation, please see our compensation resources.
 Explore the Scientific R&D Software
Explore the Scientific R&D Software