Regulated Environments
Comprehensive compliance features to meet the needs of your regulated lab
The FDA’s Electronic Records and Electronic Signatures Rule (“Part 11”) defines the requirements for submitting documentation to FDA in electronic form as well as criteria for use of electronic signatures. These regulations – which affect the creation, maintenance, transmission, storage, modification, and submission of electronic records have added new challenges to the regulated life science industries. To verify conclusions you make from test data, the FDA wants to be able to use the same tools to evaluate data that you did. The FDA also requires that you can account for every detail as to how you arrived at your results. Key to such data evaluation and manipulation is a means to control the metadata, the data that software uses to render your data into meaningful reports. FCS Express with CFR Part 11 Compliance features will help enhance your compliance program.
21 CFR Part 11 Compliance
FCS Express offers you the most complete set of 21 CFR Part 11 compliance tools to meet the needs of your regulated life-science laboratory including security, access limitations, authority checks, record protection, audit trails, electronic signatures, and much more.

User Based Security
User-Based Security allows you to restrict users from performing functions in the program. You can designate users be part of security groups that permit a defined set of the functions in FCS Express. Each user must log in with their name and password. When they try to access a restricted function, they will see an error message.

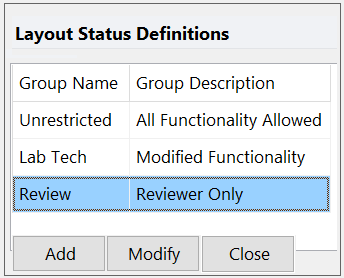
Layout Based Security
With Layout-based Security, you can set up the restrictions designated for an analysis layout. Any user who opens the layout will be prevented from performing the restricted functions allowing you to create layouts with different permissions based on your laboratories needs.
Marker, Quadrant, and Gate Locking/Visibility
Marker, quadrant, and gate editing can be set to allow move, not allow move, or allow move based on a value to "lock" down movement of these items. Additionally, visibility of markers and quadrants may be set as visible, not visible (hidden) or conditionally based on a value.
Electronic Signatures
FCS Express supports a comprehensive set of features to allow you to manage and record electronic signatures, to ensure that a member of each required group (e.g., techs, supervisors, pathologists, etc.) have viewed and approved the analysis. Signatures work in conjunction with the Security features.
Using tokens, a Signature Order can be enforced in which a signature from one particular signature group must be signed before a signature from another signature group is allowed.
Logging / Audit Trails
FCS Express has a comprehensive logging system that allows you to track every change made to any layout ensuring the integrity of your data analysis and for conforming with 21 CFR Part 11 regulations.
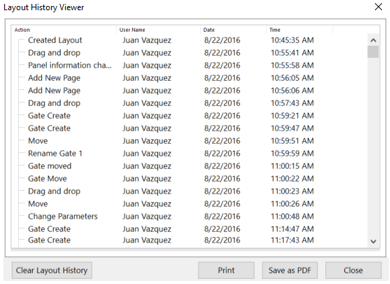
Every Detail Captured
The layout history window will show every action performed in FCS Express as part of the logging and audit trail process. Every action is linked back to the user, date and time of the event. When you are ready to report the audit trail simply print or export to PDF.
Authorizations and Permissions
All plots, text boxes, and other objects in FCS Express may be assigned Authorizations and Permission Rules at the layout level which allows or denies users from performing the actions defined. Permission Rules can be used to allow or prevent any action while Authorizations may be set to define if a user may move or edit components of the analysis.